Lithium vs. TPPL Battery Overview. Is it a fair comparison?
As lithium-ion batteries continue to grow in popularity, lead-acid battery manufacturers are now offering thin plate pure lead batteries…
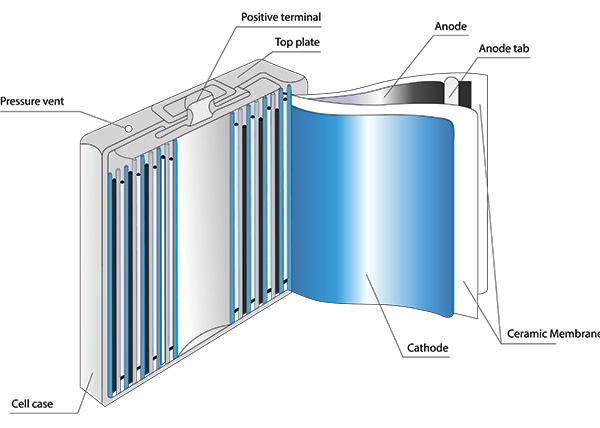
OneCharge makes the most stringent demands on the safety and quality of all components to ensure top performance and a long cycle life for our lithium batteries. We partner with the most technologically advanced, reliable manufacturers of lithium cells. We select vendors that comply with our high standards in the following three main areas of cell technology:
We work with cells with different chemistry, including LFP, NMC, and others, but focus mostly on LFP technology as the optimal choice for material-handling applications.
Electrolyte plays a key role in transporting the positive lithium ions between the cathode and anode. The most commonly used electrolyte consists of lithium salt, such as LiPF6, which is widely used in OneCharge batteries.
OneCharge advanced lithium-ion cells use high-purity electrolyte engineered to optimize the performance and longevity of the cell. It is composed of organic solvents, LiPF6 salt, and proprietary additives to improve stability and prevent dendritic formation and degradation of the solution.
These requirements are especially important in high-energy-density industrial battery applications:
There are multiple cathode electrode materials to choose from within the Li-ion technology space. Originally, the primary active component of the cathode was lithium cobalt oxide. Today, cobalt is frequently being substituted out with iron (LFP), nickel, manganese, and aluminum (NMC, NCA). Cathode materials require extremely high purity levels and must be almost entirely free of unwanted metal impurities, such as vanadium and sulfur.
Most OneCharge industrial batteries are built with LFP cells, where cathode durability is enhanced through the addition of a proprietary combination of nanoparticles.
These battery cells are engineered with anode electrodes made of natural and artificial graphite powder that is heavily processed before being baked onto copper foil. Graphite is a crystalline solid with a grey-black color and a metallic sheen. Due to its electronic structure, it is highly conductive—a single crystal can reach 25,000 siemens per square centimeter (S/cm2). The reversible electrochemical capability is maintained over several thousand of cycles in OneCharge batteries with optimized anode electrodes that are extremely light, porous and durable. The graphite surface in the cells we use is fully compatible with the rest of our lithium-ion battery chemistry—salts, solvents and binders.
Our cells are made with copper-rolled annealed foil (RA-type), made from wrought Cu, which is generally used for high-energy, high-power applications such as drivetrains in electric vehicles (EVs) and industrial batteries.
OneCharge batteries’ most demanding applications require that the cells’ anode and cathode electrodes:
An electrochemical cell consists of an anode and a cathode that are separated by an ion-permeable or ion-conductive membrane—the separator—as one of the main components. Lithium ion-conducting membranes are indispensable for long-term stable battery pack performance. The growth of metallic dendrites between the electrodes leads to potential short circuits. To minimize these risks, temperature-stable, non-flammable ceramic particles are added to the material of the composite separators.
The separator must keep anode and cathode from physically contacting each other while enabling free ionic transport. Based on the morphology of the separator, there are generally two kinds of separators, including microporous membranes and nonwoven films. Although separators are effective in preventing electrical shorts between anode and cathode, their presence between the two electrodes decreases the effective conductivity of the electrolyte, raising cell impedance. This would be expected since the presence of the separator decreases the total cross-sectional area of the lithium ion-conducting pathway, and the tortuosity of the open pores in the separator prolongs the ionic transport pathway. The thinner the separator, the higher the ionic conductivity, but there is a trade-off between the thickness of the separator and its mechanical properties.
The current state-of-the-art lithium cell technology uses a ceramic-coated separator, which improves cell performance at high temperatures and enhances battery safety.
This is the cell technology that goes into OneCharge batteries. Their membranes’ physical properties demonstrate:
As lithium-ion batteries continue to grow in popularity, lead-acid battery manufacturers are now offering thin plate pure lead batteries…
Recent independent degradation tests of commercial lithium batteries reveal a big surprise! Contrary to the claims of many NMC-based lithium…
If you’re reading this, you’re likely considering switching your fleet of forklifts and hand jacks to Lithium-Ion batteries. Or you might be…
Lithium-ion is named for its active materials; the words are either written in full or shortened by their chemical symbols. A series of…