As its name implies, “bipolar” battery technology uses an electrode that is a cathode and an anode at the same time. It is a delicate balance, as we shall see. This technology has recently reached the production stage with lead-acid batteries. Now the experts have a chance to evaluate their performance and reach a consensus:
• Lithium-ion batteries with today’s single-electrode technology demonstrate better runtime than lead-acid batteries with bipolar-electrode technology.
• At present there are only a few instances of commercial production of bipolar lead-acid batteries. Further adoption of the technology and production scalability are still uncertain.
• However, bipolar electrode technology has a chance to seriously improve battery performance if successfully implemented with high-capacity battery chemistry, such as lithium-ion.
For bipolar technology to take off, the battery components must meet certain requirements for successful commercial implementation, such as:
• excellent mechanical properties of the substrate
• very stable electrolyte in each cell without decomposition gases
• excellent electrochemical stability of the substrate at high and low voltages
• identical electron transfer during the charging/discharging procedure for each electrode
• acceptable price and easy handling of substrate materials.
It’s expected that setting up mass production and the commercialization process will take at least a decade.
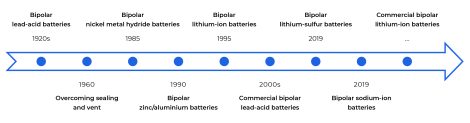
What are bipolar batteries?
The term “bipolar battery” refers to the presence of bipolar electrodes inside a battery module. Theoretically, this technology may be applied to batteries with different chemistries. In reality, among all the various bipolar batteries, only lead-acid battery modules have reached the commercial production stage. Nevertheless, it is a likely path of evolution for lithium-ion batteries, as well.
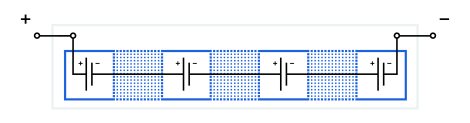
The principle of operation of a bipolar battery is quite simple—in theory. One cell’s negative electrode and another cell’s positive electrode are located very close to each other (back-to-back). The cathode and anode are both coated on the substrate. The substrate with electrodes acts as a seal for the adjacently placed cells. Electrical current flows between cells directly through both electrodes and the thin, intermediate conductive substrate. Intermediate jumpers between neighbor cells are unnecessary in this case. The cells are placed in a common container. One or more containers are united into battery modules. See below for a schematic of a bipolar electrode module.
Features of a bipolar electrode module:
- serial connection of cells (positive to negative);
- no intermediate jumpers between neighboring cells;
- a common container for cells;
- cells are isolated from each other by common substrates.
The basic idea here is to eliminate intermediate jumpers and reduce the quantity of substrates and containers. As a result, in comparison to other battery technologies, bipolar batteries can offer great advantages through:
- improving specific power;
- simplifying cell components;
- reducing manufacturing costs;
- eliminating heavy components in a battery pack.
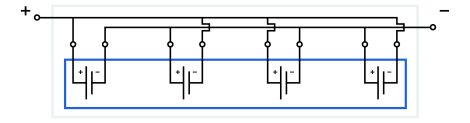
“Monopolar” electrode technology is worth mentioning here, though it has not been commercialized yet. Like bipolar technology, it, too, helps to reduce battery weight. This technology uses a reduced amount of substrate in the battery module. Imagine several individual electrode cells connected negative to negative and positive to positive instead of positive to negative, place them in a single row, and locate identical poles closer to each other (almost back-to-back). The individual cell containers may be replaced with a single common container for the whole battery module. Two directly connected electrodes may be replaced by a common two-sided electrode. A schematic of a monopolar electrode module is shown below.
A monopolar electrode module features:
- parallel connection of cells (positive to positive, negative to negative);
- intermediate jumpers between neighboring cells;
- a common container for cells.
There are no specific standards and requirements for monopolar and bipolar batteries. The number of cells in a module, cell capacity, module dimensions, and other parameters are governed by general standards that address lead-acid battery safety, performance, testing, and maintenance.
A head-to-head comparison of bipolar and monopolar cells with other types of cells cannot be done. Bipolar and monopolar cells are fully integrated in battery modules. They have no clear boundaries and cannot be repaired and replaced. These technologies’ total advantages may be seen and evaluated only at the battery module and pack levels.
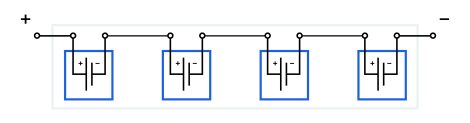
In a regular, single-electrode approach, the individual cells are self-contained. Each battery cell has its own container and has two electrodes. One cell’s electrodes are connected to another cell’s electrodes with intermediate jumpers, positive to negative. This is how cells are formed into battery modules.
A single-electrodes module features:
- serial connection of cells (positive to negative);
- intermediate jumpers between neighboring cells;
- a container for each cell.
Advantages and disadvantages of bipolar batteries
How are bipolar batteries different from today’s single-electrode batteries?
Advantages:
- Higher energy density (due to the absence of intermediate jumpers, lower weight, and smaller battery dimensions).
- Higher power density (due to a lower degree of electrode potential deviation from the equilibrium value in charging/discharging processes).
Disadvantages:
- The larger the battery module, the higher the battery replacement costs (failure of a single cell may require replacing of an entire battery array).
- More complex battery management for diagnosing cell voltage and maintaining battery health status (the whole battery system becomes abortive if any one cell fails in a multi-cell battery framework).
Why are there so few manufacturers?
As already mentioned, out of all the various bipolar batteries, only lead-acid batteries have reached the commercial production stage. This is mainly due to the very high material requirements for their components:
- Excellent mechanical properties of the substrate. The cathode and anode are both coated on the same substrate, which also serves to help seal off the electrolyte in each cell. The insufficient hardness of the substrate can lead to its rapid destruction and the failure of the entire battery module.
- Very stable electrolyte in each cell without decomposition gases. Cells cannot be allowed to swell to the detriment of electrolyte isolation. Even tiny mechanical imbalances in one cell can compromise the whole module.
- Excellent electrochemical stability of the substrate at high and low voltages. The cathode and the anode are made of different materials and are both coated on the substrate. Since different chemical reactions are happening at the same time, the system becomes less stable with voltage changes.
- Identical electron transfer during the charging/discharging procedure for each electrode. Inconsistent electrode capacity can cause over-charge and over-discharge of a cell unit. A single defective electrode in a battery module quickly kills the remaining tens of cells. Early diagnosis on the cell level is almost impossible.
- Acceptable price and easy handling of substrate materials. The current cost of a high-voltage bipolar module can exceed the cost of a regular pack with similar voltage and capacity. Moreover, for such a single-module string, partial cell group replacement is impossible.
Bipolar lead-acid battery vs. single-electrode lithium-ion battery
No head-to-head batteries comparison of bipolar lead-acid battery and single electrode lithium-ion battery has been performed yet. Fortunately, a benchmark comparison of battery modules can suffice.
The comparison demonstrates that with batteries of the same weight, bipolar lead-acid batteries are capable of providing more instant power (W), while lithium-ion single electrode modules store more electrical energy (Wh).
Speaking of material handling equipment, the heavier the equipment, the more instant power is required. And more energy is needed for longer hours of operation. In general, lithium-ion batteries with the existing single-electrode technology demonstrate better runtime.
Applications:
- The use of lead-acid batteries with bipolar electrode technology is quite limited because of the low supply of components that meet critical requirements. Nevertheless, there are several companies with operational or pilot manufacturing: Effpower for hybrid electric vehicles from Optima and Volvo; GreenSeal from ABC, Silicon Joule from Gridtential, and AACarbon from ArcActive.
- Lithium-ion batteries with single-electrode technology are mostly used in electric vehicles, energy storage, power tools, electronics, medical appliances, etc. Learn more about formats of lithium cells here: Types of lithium batteries: lithium cell formats.
 by Vladimir Karimov | December 11, 2021
by Vladimir Karimov | December 11, 2021